The following cell notation contains two half-reactions. Pt | Fe2+ (aq), Fe3+ (aq) || Sn4+ (aq), Sn²+ (aq) | Pt Which of the following statement/equation correctly describes the two half-reactions? anode: Sn4+ (aq)+2e→Sn²+ (aq); cathode: Fe2+(aq)-e→Fe³+(aq); O anode: Fe²+ (aq)-e→Fe³+ (aq); cathode: Sn²+ (aq)+2e→Sn4+ (aq) O anode: Fe³+ (aq)+e→Fe2+ (aq); cathode: Sn²+ (aq)+2e-Sn³+ (aq) anode: Pt + e → Fe³+ (aq)+Fe2+ (aq); cathode: Sn4+ (aq)+Sn²2+ (aq)+2e→Pt O anode: Fe²+ (aq)-e→Fe³+ (aq); cathode: Sn4+ (aq)+2e Sn²+ (aq)
The following cell notation contains two half-reactions. Pt | Fe2+ (aq), Fe3+ (aq) || Sn4+ (aq), Sn²+ (aq) | Pt Which of the following statement/equation correctly describes the two half-reactions? anode: Sn4+ (aq)+2e→Sn²+ (aq); cathode: Fe2+(aq)-e→Fe³+(aq); O anode: Fe²+ (aq)-e→Fe³+ (aq); cathode: Sn²+ (aq)+2e→Sn4+ (aq) O anode: Fe³+ (aq)+e→Fe2+ (aq); cathode: Sn²+ (aq)+2e-Sn³+ (aq) anode: Pt + e → Fe³+ (aq)+Fe2+ (aq); cathode: Sn4+ (aq)+Sn²2+ (aq)+2e→Pt O anode: Fe²+ (aq)-e→Fe³+ (aq); cathode: Sn4+ (aq)+2e Sn²+ (aq)
Question
100%
1a

Transcribed Image Text:The following cell notation contains two half-reactions.
Pt | Fe2+ (aq), Fe3+ (aq) || Sn4+ (aq), Sn²+ (aq) | Pt
Which of the following statement/equation correctly describes the two half-reactions?
anode: Sn4+ (aq)+2e→Sn²+ (aq); cathode: Fe2+(aq)-e→Fe³+(aq);
O anode: Fe²+ (aq)-e→Fe³+(aq); cathode: Sn²+ (aq)+2e→Sn4+ (aq)
anode: Fe³+ (aq)+e→Fe2+ (aq); cathode: Sn²+ (aq)+2e→Sn³+ (aq)
anode: Pt + e → Fe³+ (aq)+Fe2+ (aq); cathode: Sn4+ (aq)+Sn²+ (aq) +2e→Pt
O anode: Fe²+ (aq)-e→Fe³+ (aq); cathode: Sn4+ (aq)+2e-Sn²+ (aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Using data provided in the table of Standard Potentials, calculate standard emf of the cell described in the last question. Please enter your answer with 2 decimals. for example, 1.056 is written as 1.06. Pay attention to the sign.
this is in par to this question thread
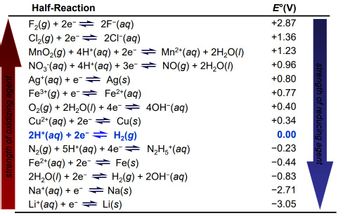
Transcribed Image Text:Half-Reaction
F₂(g) + 2e
2F-(aq)
Cl₂(g) + 2e
2C1-(aq)
MnO₂(g) + 4H+ (aq) + 2e¯ ⇒
NO3(aq) + 4H+ (aq) + 3e¯ ⇒
Ag(s)
Mn²+ (aq) + 2H₂O(l)
NO(g) + 2H₂O(l)
Ag+ (aq) + e
Fe³+ (g) + e
Fe²+ (aq)
O₂(g) + 2H₂O(l) + 4e¯ ⇒ 4OH¯(aq)
Cu²+ (aq) + 2e → Cu(s)
2H*(aq) + 2e = H₂(g)
N₂H5*(aq)
N₂(g) + 5H+(aq) + 4e¯⇒
Fe²+ (aq) + 2e → Fe(s)
2H₂O(l) + 2e → H₂(g) + 2OH(aq)
Na+ (aq) + e
Na(s)
Li+ (aq) +eLi(s)
E°(V)
+2.87
+1.36
+1.23
+0.96
+0.80
+0.77
+0.40
+0.34
0.00
-0.23
-0.44
-0.83
-2.71
-3.05
strength of reducing agent
Solution
Follow-up Question
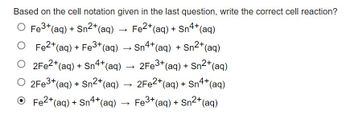
Transcribed Image Text:Based on the cell notation given in the last question, write the correct cell reaction?
O Fe³+ (aq) + Sn²+ (aq)
O Fe²+ (aq) + Fe3+ (aq)
O 2Fe2+ (aq) + Sn4+ (aq)
O 2Fe3+ (aq) + Sn²+ (aq)
Fe2+ (aq) + Sn4+ (aq)
Fe2+ (aq) + Sn4+ (aq)
› Sn4+ (aq) + Sn2+ (aq)
2Fe3+ (aq) + Sn2+ (aq)
2Fe2+ (aq) + Sn4+ (aq)
Fe3+ (aq) + Sn²+ (aq)
O
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.